[ad_1]
The world of AI-powered drug discovery retains increasing because the capabilities of machine studying develop. One strategy that appeared unthinkable only a few years in the past is simulating the sophisticated interplays of two interlocking molecules — however that’s precisely what drug designers must learn about, and precisely what Charm Therapeutics goals to do with its DragonFold platform.
Proteins do nearly every thing value doing in your physique, and are essentially the most frequent targets for medication. And so as to create an impact, it’s essential to first perceive that focus on, particularly how the chain of amino acids making up the protein “folds” below totally different circumstances.
Within the latest previous this was usually carried out with complicated, time-consuming X-ray crystallography, however it has lately been proven that machine studying fashions like AlphaFold and RoseTTAFold are able to producing outcomes simply pretty much as good however in seconds fairly than weeks or months.
The subsequent problem is that even when we all know how a protein folds in its commonest situations, we don’t know the way it would possibly work together with different proteins not to mention novel molecules made particularly to bind with them. When a protein meets a suitable binder or ligand, it could actually remodel fully, since small modifications can cascade and reconfigure its complete construction — in life this results in issues like a protein opening a passage right into a cell or exposing a brand new floor that prompts different proteins, and so forth.
“That’s actually the place we’ve got innovated: we’ve got constructed DragonFold, which is the primary protein-ligand co-folding algorithm,” mentioned Laskh Aithani, CEO and co-founder of Appeal Therapeutics.
“Designing medication that bind to the disease-causing protein of curiosity very tightly and selectively (i.e., keep away from binding to different related proteins which can be required for regular human functioning) is of paramount significance,” he defined. “That is carried out most simply when one is aware of how precisely these medication bind to the protein (the precise 3D form of the ligand sure to the disease-causing protein). This permits one to make precision modifications to the ligand such that it could actually bind extra tightly and extra selectively.”
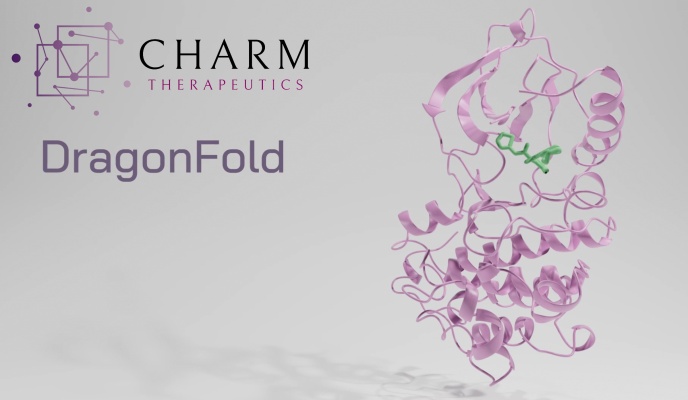
You may see a illustration of this case on the high of the article: The small inexperienced molecule and the purple protein match collectively in a really particular method that isn’t essentially intuitive or simple to foretell. Efficient and environment friendly simulation of this course of helps display screen billions of molecules, much like earlier processes that recognized drug candidates however going additional and lowering the necessity to experimentally verify whether or not they work together as anticipated.
To perform this, Aithani tapped David Baker, designer of the RoseTTAFold algorithm amongst many others and head of an influential lab at the University of Washington, to be his co-founder. Baker is well-known in academia and trade as one of many main researchers on this space, and he has revealed quite a few papers on the topic.
Appeal Therapeutics co-founders Laskh Aithani (left) and David Baker. Picture Credit: Appeal Therapeutics
Shortly after it was proven that algorithms might predict protein buildings primarily based on their sequence, Baker established they may additionally “hallucinate” new proteins that acted as anticipated in vitro. He’s very clearly on the vanguard right here. And he gained a $3 million Breakthrough prize in 2020 — undoubtedly as much as being a technical co-founder. Aithani additionally proudly famous the presence of DeepMind veteran Sergey Bartunov as director of AI and former pharma analysis lead Sarah Skerratt as head of drug discovery.
The $50 million A spherical was led by F-Prime Capital and OrbiMed, with participation from Normal Catalyst, Khosla Ventures, Braavos and Axial. Whereas such giant quantities should not unusual for software program startups, it needs to be famous that Appeal isn’t stopping at constructing the potential of characterizing these protein-ligand interactions.
The corporate’s early-stage funding was used to construct the mannequin, however now they’re shifting on to the following step: constructive identification of efficient medicines.
“We’ve got the preliminary model [of the model] prepared, and that has been validated in-silico,” Aithani mentioned. “Over the approaching quarters, we’re validating it experimentally. Observe that the ‘product’ will primarily be for inside use to assist our personal scientists uncover potential medicines that we personal 100% of the rights to.”
Ordinarily the testing course of entails wet-lab screening of 1000’s upon 1000’s of candidate molecules, but when it really works as marketed, DragonFold ought to massively minimize down on that quantity. Which means a comparatively small lab with a comparatively small price range can conceivably house in on a drug that a couple of years in the past would possibly require a significant pharma firm investing a whole bunch of hundreds of thousands.
Contemplating the revenue profile of a novel drug, it’s no shock that the corporate has attracted this type of funding: a couple of tens of hundreds of thousands is a drop within the bucket in contrast with the R&D price range of any massive biotech analysis firm. All it takes is one hit and so they’re laughing. It nonetheless takes some time, however AI drug uncover shortens timelines as nicely — so count on to listen to about their first candidates sooner fairly than later.
[ad_2]
Source link